I was down with a fever a few days ago, so just popped out a tablet of paracetamol from the strip and asked my mother to get a glass of water. It took her a few minutes to get it and I just looked at the tablet as I held it in on my palm. That’s when a thought struck me – ‘How are these medicines even discovered and how are they designed to treat a specific disease?’. This curiosity kept ringing at the back of my head. A couple of days after the fever subsided, I made it a point to browse about the process of discovery of medicines. The journey of a medicine (referred to as a ‘drug’ in pharmacology) from the laboratory – ‘SLAB’ to the market – ‘SHELF’.
My study about the drug development process started off with astonishment as I read that it takes about 12-15 years, which is approximately 1/3rd of the professional career of an individual!!! at a cost of about $1 Billion! to get a drug approved for clinical use.
Before a drug reaches the patient, it is important to determine its safety and efficacy including the exact dosage and an appropriate route of administration, through numerous tests.
Pharmaceutical regulatory authorities monitor the entire drug development flow and are responsible to ensure the safety, efficacy, accessibility and security of approved drugs.
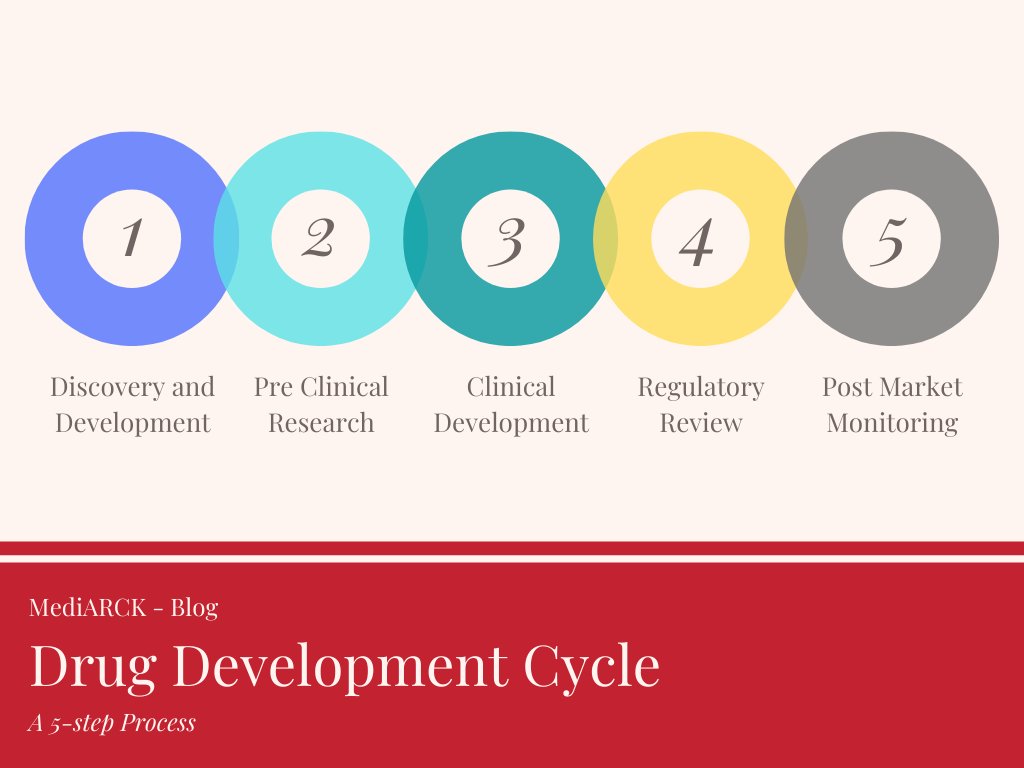
The drug development process includes five main steps:
Step 1: Discovery & Development
Step 2: Preclinical Research
Step 3: Clinical Development / Clinical Research
Step 4: Regulatory Review
Step 5: Post-marketing surveillance
As I was a bit determined to study the whole anecdote of drug discovery, I decided to wade through each step in detail, but on the next day!

